

The Duke-UNC Alzheimer’s Disease Research Center aims to catalyze research, innovations in clinical care and academic work force development with UNC Pembroke as a partner institution
- UNCP research scientist Ben Bahr earns top mentoring award (2021)
- UNCP’s landmark patent to aid in Alzheimer’s disease, TBI treatment (2020)
- Ben Bahr is named the Oliver Max Gardner Award Winner (2017)
- Professor Ben Bahr is featured in Healthcare Audio Podcast (2016)
- Professor Ben Bahr receives UNC Board of Governors' James E. Holshouser, Jr., Award for Excellence in Public Service (2013)
- Watch profile of Ben Bahr (aired on UNC TV's North Carolina Now: Nov. 11, 2013)
- Watch YouTube video: Ben Bahr on Understanding Alzheimer's Disease (UNCP)
The Amazing Brain and How it Strives to Fight Off Dementia and Injuries
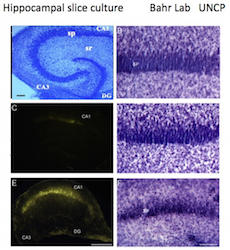
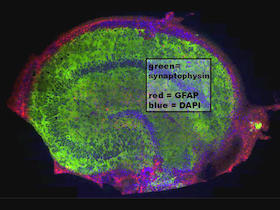
The >500,000 gigabyte hard drive floating in your skull is a huge challenge to study, the brain being the most complicated memory-encoding machine known. In the Bahr Lab, researchers maintain brain explants in culture for months to investigate vulnerable neuronal connections that are responsible for learning, memory, and creativity. Bahr’s team focuses on synaptic vulnerability that contributes to dementia risk factors, thereby improving our understanding of the synaptopathy initiated by seizure- and stroke-type excitotoxicity, TBI, military blast exposures, and brain aging – all linked to elevated risks of dementia. The research integrates explant models with transgenic animal models to study different dementias, using cell signaling, bioinformatics, and drug design methods to determine pathogenic cascades involved and to identify repair mechanisms and therapeutic strategies against the mild cognitive impairment (MCI) to dementia continuum.
Brief Bio: Dr. Bahr is the William C. Friday Chair and Professor at UNCP. His Ph.D. in chemistry from University of California–Santa Barbara identified a target for the diagnosis of Alzheimer’s disease, the most common form of dementia. His postdoctoral training was at the Center for the Neurobiology of Learning and Memory, University of California–Irvine. He has presented his team’s research in 18 countries, has over 150 publications and patents, and leads UNCP as a partner institution of the Duke-UNC Alzheimer’s Disease Research Center. His honors include several mentoring awards for his work with postdocs, graduate students, and invaluable undergraduate researchers who received NSF, Glaxo Women in Science, NCBiotech, and NC Space/NASA Grant Scholarships, NIH RISE Fellowships, NCBiotech Internships, and the Timothy Ritter and Marie Amero Endowed Research Scholarship.

Members of the William C. Friday Laboratory
- Ben A. Bahr, Ph.D. (PI; Depts. of Biology and Chemistry & Physics)
- Kinsley Adams, B.S. (Research Specialist / Lab Manager)
- Michael Almeida, Ph.D. (Duke-UNC ADRC REC Scholar at UNC-Chapel Hill; UNCP Adjunct Research Associate)
- Karen Farizatto, Ph.D. (Duke-UNC ADRC REC Scholar; UNCP Adjunct Research Associate)
- Naomi Fountain; Biology (Honors College Project)
- Reece Hicks; Chemistry (NCBiotech Intern, Timothy Ritter and Marie Amero Endowed Research Scholarship)
- Mykayla Green; Chemistry (NCBiotech Intern)
- Irayza Grijalva; Biology
- Kani McRae; Molecular Biology
- Ayed Abudayya; Biology/Genetics
- Reagan Davidson; Biology
- Alexis Jones; Biology
- Julissa Enriquez; Biology
- Asil Abudayeh; Biology
Recent Publications (from list of 155)
(click here for publications available via NCBI)
Almeida MF, Piehler T, Carstens KE, Zhao M, Samadi M, Dudek SM, Norton CJ, Parisian CM, Farizatto KLG, and Bahr BA (2021) Distinct and dementia-related synaptopathy in the hippocampus after military blast exposures. Brain Pathology 31:e12936. doi.org/10.1111/bpa.12936
Caba E, Sherman MD, Farizatto KLG, Alcira B, Wang H-W, Giardina C, Shin D-G, Sandefur CI, and Bahr BA (2021) Excitotoxic stimulation activates distinct pathogenic and protective expression signatures in the hippocampus. J Cell Mol Medicine 00:1–17. doi.org/10.1111/jcmm.16864
Almeida MF, Bahr BA, and Kinsey ST (2020) Endosomal-lysosomal dysfunction in metabolic diseases and Alzheimer’s disease. Internatl Review Neurobiol 154:303-324.
Telpoukhovskaia MA, Liu K, Sayed FA, Iker Etchegaray J, Xie M, Zhan L, Li Y, Zhou Y, Le D, Bahr BA, Bogyo M, Ding S, and Gan L (2020) Discovery of small molecules that normalize the transcriptome and enhance cysteine cathepsin activity in progranulin-deficient microglia. Scientific Reports 10:13688. doi.org/10.1038/s41598-020-70534-9
Farizatto KLG, Almeida MF, Long RT, and Bahr BA (2019) Early synaptic alterations and selective adhesion signaling in hippocampal dendritic zones following organophosphate exposure. Scientific Reports 9:6532.(nature.com/articles/s41598-019-42934-z).
Hwang J, Estick CM, Ikonne US, Butler D, Pait MC, Elliott LH, Ruiz S, Smith K, Rentschler KM, Mundell C, Almeida MF, Stumbling Bear N, Locklear JP, Abumohsen Y, Ivey CM, Farizatto KLG, and Bahr BA (2019) The role of lysosomes in a broad disease-modifying approach evaluated across transgenic mouse models of Alzheimer's disease and Parkinson's disease and models of mild cognitive impairment. International J Mol Sci 20:4432. **In Special Issue Biogenesis and Functional Roles of Lysosomes: Their Implications for the Pathogenesis and Therapy of Human Diseases. LINK
Kakoki M, Bahnson EM, Hagaman JR, Siletzky RM, Grant R, Kayashima Y, Li F, Lee EY, Sun MT, Taylor JM, Rice JC, Almeida MF, Bahr BA, Jennette JC, Smithies O, and Maeda-Smithies N (2019) Engulfment and cell motility protein 1 potentiates diabetic cardiomyopathy via Rac-dependent and Rac-independent ROS production. J Clin Invest Insight 4:e127660. LINK
Lamani M, Malamas MS, Farah SI, Shukla VG, Almeida MF, Weerts CM, Anderson J, Wood JT, Farizatto KLG, Bahr BA, and Makriyannis A (2019) Piperidine and piperazine inhibitors of fatty acid amide hydrolase targeting excitotoxic pathology. Bioorganic Med Chem 27:115096. doi.org/10.1016/j.bmc.2019.115096
Wang C, Telpoukhovskaia MA, Bahr BA, Chen X, and Gan L (2018) Endo-lysosomal dysfunction: a converging mechanism in neurodegenerative diseases. Current Opinion Neurobiol 48:52-58. LINK
Farizatto KLG, Ikonne US, Almeida MF, Ferrari MFR, and Bahr BA (2017) Aβ42-mediated proteasome inhibition and associated tau pathology in hippocampus are governed by a lysosomal response involving cathepsin B: Evidence for protective crosstalk between protein clearance pathways. PLoS ONE 12:e0182895. LINK
Farizatto KLG, McEwan SA, Naidoo V, Nikas SP, Shukla VG, Almeida MF, Byrd A, Romine H, Karanian DA, Makriyannis A, and Bahr BA (2017) Inhibitor of endocannabinoid deactivation protects against in vitro and in vivo neurotoxic effects of paraoxon. J Mol Neuroscience 63:115-122. LINK
Parisian CM, Georgevitch G, and Bahr BA (2017) Military blast-induced synaptic changes with distinct vulnerability may explain behavioral alterations in the absence of obvious brain damage. J Nature Sci 3:e406.
Carrasco DI, Bahr BA, Seburn KL, and Pinter MJ (2016) Abnormal response of distal Schwann cells to denervation in a mouse model of motor neuron disease. Exp Neurol 278:116-126. LINK
Smith M, Piehler T, Benjamin R, Farizatto KL, Pait MC, Almeida MF, Ghukasyan VV, and Bahr BA (2016) Blast waves from detonated RDX explosive reduce GluR1 and synaptophysin levels in hippocampal slice cultures. Exp Neurol 286:107-115. LINK
Maltecca F, Baseggio E, Consolato F, Mazza D, Podini P, Young SM Jr, Drago I, Bahr BA, Puliti A, Codazzi F, Quattrini A, and Casari G (2015) Purkinje neuron Ca2+ influx reduction rescues ataxia in the spinocerebellar ataxia type 28 (SCA28) model. J Clin Invest 125:263-274. LINK
Bahr BA (2014) A single pathway targets several health challenges of the elderly. Rejuvenation Research 17:382-384.
Previous Undergraduate Researchers (RISE Fellows*; COMPASS Scholars#):
- Joanna Cooper* (NCBC Fellow)
- Hollie Young* (Gordon Conference Carl Storm Fellow)
- David Blake
- Emily Graves*
- Christopher Visser
- Rebecca Howell* (NCBC Fellow)
- Daisy Irra*
- Jasmine Robinson*
- Sarah Gambrel*
- Josie Torrence*
- Vivian Anunobi*
- Arieana Van Allen
- Tyler Loehr*
- Katharine Willoughby (Hawk Fellow)
- Jody Long
- Jasmine Rowlett*
- Ginny Holland
- Thomas Romine (Research & Engineering Apprenticeship)
- Johnathan Locklear*
- Pamela Marie Quizon (Research Assistant)
- Davita Brockington
- Ye Lin (undergrad research assistant)
- Elizabeth Metzger (NC Space Grant/NASA Scholar)
- Robert Baldi
- Kassie Conway*
- Jordon Smink*
- Louis Leonard
- Paul Freeman*
- M. Tyler Bullock (Engineering Apprenticeship) Robeson Early College
- Sarah Hafner-Ruiz* (NC Space Grant/NASA Scholar)
- Olivia Bullard* (Glaxo Scholar Women in Science)
- Marsalis Smith*
- Armando Corona*
- Julia McGee*
- Samantha Suggs
- Rebecca Jackson
- Veronica Robles
- Emily Suzanne Pope
- Kayla J. Oxendine
- Sonny Ruiz
- Chaterria Perry*
- Darian Duval; Biology and Psychology
- Joshua Ellerbe
- Benson Mbugua
- Kathlyn Stephens (PURC Research Fellow)
- Morgan C. Pait*
- Lyndsie Elliott (PURC Research Fellow)
- Justin Showers
- Matt MacDougall; Chemistry
- Dalton Brooks
- Christopher Long*
- Justin Floyd
- Marcus Sherman*
- Ziporia Swift*
- Aaron Byrd*
- Sara A. McEwan*
- Marilyn Louise Knotts
- Paul J. Lascuna*#
- Melissa Teel
- Katrina Dogramatzis; Brunswick Comm. College
- Michael Nance
- Chelsea Prevatte
- Jonathan Malcolm; Engineering Apprenticeship Program (Army)
- Jeffrey MacDonald
- Jerronatan Chavis; 8th grader
- Sushant Koirala; Early College Summer Program
- Cary Mundell* (PURC Research Fellow)
- Justin Branch*
- Katherine Rentschler*# (Glaxo Women in Science Scholar)
- Walter Patterson*; Chemistry
- Hailey Kelbaugh* (PURC Research Fellow)
- Donna Porter* (PURC Research Fellow)
- Kellie Chapman; Biology
- Amanda Taylor
- Emem Warrie; Nursing Program
- Trae Griffin
- Divine K Mbah
- Natasha Wells*; Chemistry
- Cora Bright*
- Kara Stomp
- Tamille Rhynes*
- Amanda Bowman
- Dea'vion Godfrey (Robeson Early College; Lab Tech Intern)
- Jasmine Sanderson
- Kaitlan Smith*#; COMPASS Scholar and Summer Rise Scholar
- Ronald Long; Summer Rise Scholar
- Michael Joe Blair
- Victor Cole
- Sports Tweede
- Katelynn Jacobs
- Iliana Claudio; Robeson Early College (Senior Internship)
- Ayanna Edwards*
- Alyssa Norton
- Camille Colvin
- Ross Masters
- Yara Abumohsen
- Swathi Gadi
- Nathan Jennings
- Brenna Sifford
- Frederick Feely II
- Jullienne Lim (Honors College)
- Jessica Rice (Augustine Rice)*
- Christopher Norton; Summer Rise Scholar
- Marica Thomas*
- Britney Alcira
- Georgian B Kailondo
- Valerie A Odugba
- Marianna Coppola
- Aseel Abumohsen
- Rawon Abumohsen
- Asil Abudayeh
- Sean Griffith
- Taylor Williams
- Garrett Barnett
- Cheyenne Lee
- Zarria Synphony Ray
- Nicole Stumbling Bear (NASA/NC Space Grant Teacher Scholarship)
- Sandra Huneycutt*
- Erica Baynard*
- Patrick Britt (Applied Physics and Mathematics)
- Caitlan McKenzie
- Jarirus Salmon (Forensic Chemistry)
- Crystian Amaya
- Rachel Maynor
- Joselyn Salmeron
- Minh Huy Giang* (ACS Organic Chem Award)
- Malinda Jolly (NAACP Chapter President)
- Tyra Critchley (Honors College)
- Ethan Williamson (Honors College Project)
- Rachel Rigter
- Anna Jones
- Christian Oxendine (Pembroke Middle School)
- Fredejah Royer*
- Maleeha Hassan
- Jeremy Hunt
- Sandrena Nalls
- James Locklear (UNCP Biotechnology Internship)
- Tierra Fields
- Caitlyn Young* (Dr. Farizatto mentor)
- Alexis Fields
- Aaron Clark
- Nysja Campbell-Smalls*
- Matthew Montilus
- Kaitlyn Nelson (Honors College Project)
- Jared Tuton (NASA/NC Space Grant Scholar)
- Mercedes Dos Santos
- Morgan Casanova
- Zharia Sims-Edwards
- Nathan London
- Alexandra Moore
- Jessalyn Nguyen*
- Nicholas Willard* (co-mentored with Dr. Poage)
- Nikki Sidney Clayman (NASA/NC Space Grant Scholar)
- Amy Stanek
- Ashton Tillett
- Kani McRae
- Kylee Crabtree
- Dean Foggan
- Mark Yorio
- Shelby Ashford
- Naomi Fountain (Honors College Project)
- Kinsley Adams (co-mentored with Dr. Farizatto)
- Jesenia Waters; (co-mentored with Dr. Farizatto)
- Iraysa Grijalva (co-mentored with Drs. Farizatto & S. Smith)
- Morgana Viana (co-mentored with Dr. Farizatto)
- Glorimar Cruz-Pagan (co-mentored with Dr. Farizatto)
- Ashley Edwards (NASA/NC Space Grant Scholar)
- Baniz Zangana
- Mikayla Williams
- Haleigh Wooters
- Danielle Topper (Harrison High School, NY; co-mentored with Dr. Smith)
- Jordan Locklear
- Esmeralda Sanchez
- Aaron Bonner-Wright
- Grant Gabzdyl
- Sydney Allen
- Karina Avila
- Jacqueline Swann
- Justin Cornelison (co-mentored with Dr. Poage)
- Ayed Abudayya
- Alexis Jones
- Reagan Davidson
- Reece Hicks #
- Julissa Enriquez
- Asil Abudayeh
- Mykayla Green
- Genesis Gregory
- Mark Yorio
Current Funding
NIH Duke-UNC Alzheimer’s Disease Research Center (ADRC) 2021-2026 UNCP partner, member of the Research & Education Core/Component (REC)
Focus: To Understand Age-Related Factors Across the Lifespan that Influence Development, Progression, and Experience of Alzheimer’s Disease
2022 BioImaging North America (BINA) Grant Role: PI (Almeida co-PI)
Title: Imaging Ideas for Undergraduate Research Scientists “Supported in part by BioImaging North America”
2022 UNCP Faculty Research Grant Role: PI
Title: Proteome-scale Assessment of Brain Vulnerabilities to Identify Biomarkers of Early Dementia
xTechHBCU-1 (Dept. of Defense HBCU/MI Research Program) 2022 Role: PI
Title: Cognitive Health Maintained through Components of the Autophagy-Lysosomal Pathway Aims: To identify i) cellular elements that contribute to age-related proteostatic stress and ii) adaptive components of autophagic-lysosomal signaling, both to disrupt the link to dementia risk.
W911NF-14-2-0087 Add-On Grant US Army Res Office 2020-2023 Role: PI
Research Grant: Glial Changes Associated with RDX Blast-Induced Effects in Brain Tissue
Aims: This will assess glial changes after blast trauma in hippocampus.
W911NF-21-1-0238 US Army Research Office Role: PI
Grant: High-Resolution Microscopy System for Training Under-Represented Minority Scholars.
Aims: The imaging system will provide acquisition of details describing i) intracellular alterations, ii) interactions between cells, as well as iii) adhesion dynamics underlying signal transduction between extracellular matrices and cytoskeletal components.
W911NF-15-1-0432 Dept. of Defense Research & Education Program
Research Grant: The role of astrocyte activation in anticholinesterase-induced synaptic changes and behavioral deficits.